The basic rules in any good manufacturing practice (GMP)
regulations specify that the pharmaceutical manufacturer must maintain proper
documentation and records.
Documentation helps to build up a detailed picture of what a
manufacturing function has done in the past and what it is doing now and, thus,
it provides a basis for planning what it is going to do in the future.
Regulatory inspectors, during their inspections of manufacturing sites, often
spend much time examining a company’s documents and records.
Data may be generated by
(i)
a paper-based record
of a manual observation, or
(ii)
in terms of equipment,
a spectrum of simple machines through to complex highly configurable
computerized systems. The inherent risks to data integrity may differ depending
upon the degree to which data (or the system generating or using the data) can
be configured, and therefore potentially manipulated
Systems should be designed in a way that encourages
compliance with the principles of data integrity.
• Access to clocks for recording timed events
• Accessibility of batch records at locations where
activities take place so that ad hoc data recording and later transcription to
official records is not necessary
• Control over blank paper templates for data recording
• User access rights which prevent (or audit trail) data
amendments
• Automated data capture or printers attached to equipment
such as balances
• Proximity of printers to relevant activities
• Access to sampling points (e.g. for water systems)
• Access to raw data for staff performing data checking
activities.
In recent years there has been a significant
increase in the number and types of data integrity issues that have been cited
in regulatory inspections by both USA and European (especially MHRA)
investigators. In fact, data integrity issues have been increasing to become
one of the most important GMP issues. This increase has led to the FDA
establishing the types of issues that should be considered red flags triggering
a more intensive investigation. They are also concerned with the impact of the
data integrity problems on the firm and the products it manufactures.
To avoid such type of issue in our
organization, ALCOA must be understood & considered as a part of our daily
life.
The Data Quality Equation
Regulatory agencies around the globe are focused on assuring patient safety and product quality; if we focus on the latter, product quality, many regulations and guidance identify the elementary expectations to achieve it. Product quality is derived from quality data that supports or gives evidence to the quality of the product. Recent regulatory observations direct industry to the conclusion that there can be severe penalties for not having data quality that leads to product quality.
What is Data Quality and how do we achieve it?
Data Integrity provides data we can trust and it is also the foundation of the Data Quality Equation. Data Management is the process by which we create, control, manage, utilize and maintain our data’s integrity. The combination of Data Integrity and Data Management results in Data Quality. In other words, Data Quality is mutually dependent on both Data Integrity and Data Management. Subsequently, Data Quality can be represented as:
Data Integrity + Data Management = Data Quality
Another element of this equation that should be considered is that better Data Integrity and/or better Data Management would produce better Data Quality. For example, if a more efficient means for Data Management can be created that eliminates risk and adds value to the process you can in turn realize fewer mistakes and higher Data Quality. Conversely, Data Quality with Data Integrity but without Data Management lacks control of your data. Similarly Data Management without Data Integrity will lack the elements necessary to have Data Quality.
Data Integrity can be expressed in ALCOA+ elements, where the acronym stands for Attributable, Legible, Contemporaneous, Original, Accurate, complete, consistent enduring and available. Data Integrity is achieved when all ALCOA+ elements are present.
The Data Quality Equation
Regulatory agencies around the globe are focused on assuring patient safety and product quality; if we focus on the latter, product quality, many regulations and guidance identify the elementary expectations to achieve it. Product quality is derived from quality data that supports or gives evidence to the quality of the product. Recent regulatory observations direct industry to the conclusion that there can be severe penalties for not having data quality that leads to product quality.
What is Data Quality and how do we achieve it?
Data Integrity provides data we can trust and it is also the foundation of the Data Quality Equation. Data Management is the process by which we create, control, manage, utilize and maintain our data’s integrity. The combination of Data Integrity and Data Management results in Data Quality. In other words, Data Quality is mutually dependent on both Data Integrity and Data Management. Subsequently, Data Quality can be represented as:
Data Integrity + Data Management = Data Quality
Another element of this equation that should be considered is that better Data Integrity and/or better Data Management would produce better Data Quality. For example, if a more efficient means for Data Management can be created that eliminates risk and adds value to the process you can in turn realize fewer mistakes and higher Data Quality. Conversely, Data Quality with Data Integrity but without Data Management lacks control of your data. Similarly Data Management without Data Integrity will lack the elements necessary to have Data Quality.
Data Integrity can be expressed in ALCOA+ elements, where the acronym stands for Attributable, Legible, Contemporaneous, Original, Accurate, complete, consistent enduring and available. Data Integrity is achieved when all ALCOA+ elements are present.
Use of ALCOA+
In recent
years there has been a significant increase in the number and types of data
integrity issues that have been cited in regulatory inspections by both USA and
European (especially MHRA) investigators. In fact, data integrity issues have
been increasing to become one of the most important GMP issues. This increase
has led to the FDA establishing the types of issues that should be considered
red flags triggering a more intensive investigation. They are also concerned
with the impact of the data integrity problems on the firm and the products it
manufactures.
To avoid
such type of issue in our organization, ALCOA must be understood &
considered as a part of our daily life.
The acronym ALCOA has been
widely associated with data integrity by FDA and was first used by Stan Woollen
when he worked for the agency to help him remember compliance terms relevant to
data quality (6). The good automated manufacturing practice (GAMP) guide “A
Risk-Based Approach to GxP Complaint Laboratory Computerized Systems” (7)
includes an appendix (Appendix 3) on data integrity. The terms used in the
appendix are sometimes referred to as “ALCOA +” because they incorporate
additional terms based on the European Medicines Agency’s concept paper on
electronic data in clinical trials (8). The terms associated with ALCOA + are
described
Below
are the some observations from warning letters, related with ALCOA term.
- HPLC & Gas Chromatography (GC) computer software lacked active audit trail functions to record changes to data, including information on original results, the identity of the person making the change, and the date of the changes.
- At least five HPLCs were used with software audit trail function not enabled, resulting in the fact that raw data sample sets could not be satisfactorily verified.
- GC software lacked active audit trail functions to record any changes to the data, including the previous entries, who made the changes & when the change were made.
- Use of correction tape over multiple entries of raw material batch number in a log book.
- The audit trail feature for your gas chromatography (GC) instruments was not used until October 2013, even though your 2009 GC software validation included a satisfactory evaluation of the audit trail capability.
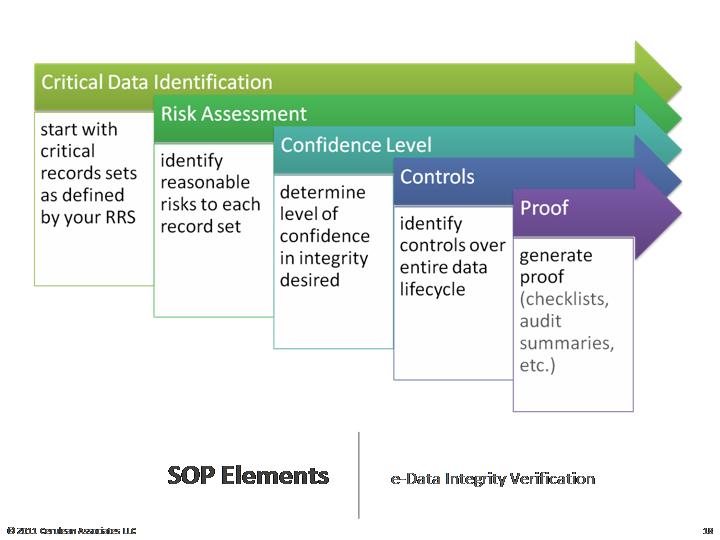
Key in
detecting & preventing issues on Data Integrity are answers to some of the
fundamental questions listed below:
1. Do mechanisms exist to ensure data is
authentic, retrievable?
2. Where critical data are being generated
manually, is there an additional check on the accuracy of the entry?
3. Is the data traceable and or referenced
to original raw data and review by a reliable quality structure?
4. Do the computerized systems used have
sufficient controls to prevent unauthorized access or changes to data and audit
trials?





No comments:
Post a Comment