About HEPA (High efficiency particulate air)
High-Efficiency Particulate Air or HEPA
is a type of air filter. It is a type of filter which has high
efficiency to filter out about 99.997% that is why they are termed as
High efficiency particulate air filter,HEPA filters are made up of a
mesh of fiber glass fibers of thickness ranging from 0.5 to 2 micrometer
and are closely packed with each other with as low clearance as 0.3μm
which may be bit greater.
Particles in air are trapped in to HEPA
filter fibers when air caring these particles is blown or passed through
these fibers, by mechanism of interception (particles adhere to
fibers), impaction (particles embed in to the gap) and diffusion and
then blocking, particles with lower particle size than the gap between
these fibers are also trapped as the result above mechanisms fiber
diameter, filter thickness, and face velocity are the factors which
affect the efficacy of the HEPA filter.
These particles are trapped (they stick to a fibre) through a combination of the following three mechanisms:
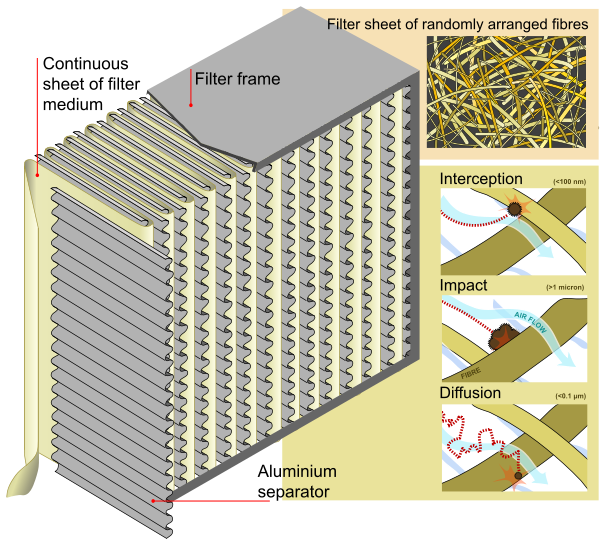 |
| HEPA-Filter with functional description |
- Interception, where particles following a line of flow in the air stream come within one radius of a fibre and adhere to it.
- Impaction, where larger particles are unable to avoid fibres by following the curving contours of the air stream and are forced to embed in one of them directly; this effect increases with diminishing fibre separation and higher air flow velocity.
- Diffusion, an enhancing mechanism that is a result of the collision with gas molecules by the smallest particles, especially those below 0.1 µm in diameter, which are thereby impeded and delayed in their path through the filter; this behaviour is similar to Brownian motion and raises the probability that a particle will be stopped by either of the two mechanisms above; it becomes dominant at lower air flow velocities.
Importance of HEPA filter in
pharmaceutical industry very great, HEPA filters are used in industry
where ever it is require to make the environment free of particulate
matter as well as free from microbial contaminants (bacterial ,viruses
etc) in clean rooms
A HEPA Filter of efficiency of 99.97% is usually used in aseptic manufacturing filing and packing, parentral dosage manufacturing.These filters are mounted in Laminar Air Flow Bench which are then used as clean area for actual aseptic manipulations or aseptic filling where usually a clean area of class 100 are required, such laminar air flow bench are typically found within a clean area of class 1000, which again are maintained by HEPA filters installed in a HVAC system.
A HEPA Filter of efficiency of 99.97% is usually used in aseptic manufacturing filing and packing, parentral dosage manufacturing.These filters are mounted in Laminar Air Flow Bench which are then used as clean area for actual aseptic manipulations or aseptic filling where usually a clean area of class 100 are required, such laminar air flow bench are typically found within a clean area of class 1000, which again are maintained by HEPA filters installed in a HVAC system.
For High Efficiency Particulate Air filter , A velocity of 0.45
meters/second (90 feet per minute) has generally been established, with a
range of plus or minus 20 percent around the setpoint. Higher
velocities may be appropriate in operations generating high levels of
particulates.
High-Efficiency Particulate Air (HEPA) efficacy test and test for integrity.
An intact HEPA filter should be capable
of retaining at least 99.997 percent of particulates greater than 0.3 nm
in diameter. The filters maximum resistance to airflow, or pressure
drop, at its nominal flow rate is usually specified around 300 Pa.
Leak test of HEPA filter
High-Efficiency Particulate Air (HEPA) filter integrity should be maintained to ensure aseptic conditions. Leak testing should be performed at installation to detect integrity breaches around the sealing gaskets, through the frames, or through various points on the filter media. Thereafter, leak tests should be performed at suitable time intervals for HEPA filters in the aseptic processing facility.
High-Efficiency Particulate Air (HEPA) filter integrity should be maintained to ensure aseptic conditions. Leak testing should be performed at installation to detect integrity breaches around the sealing gaskets, through the frames, or through various points on the filter media. Thereafter, leak tests should be performed at suitable time intervals for HEPA filters in the aseptic processing facility.
For example, such testing should be
performed twice a year for the aseptic processing room. Additional
testing may be appropriate when air quality is found to be unacceptable,
facility renovations might be the cause of disturbances to ceiling or
wall structures, or as part of an investigation into a media fill or
drug product sterility failure. Among the filters that should be leak
tested are those installed in dry heat depyrogenation tunnels and ovens
commonly used to depyrogenate glass vials. Where justified, alternate
methods can be used to test HEPA filters in the hot zones of these
tunnels and ovens.
What is meant terms Efficiency, Penetration,Most Penetrating Particle Size (MPPS), for a HEPA filter
Penetration :
Penetration is the ratio of the particle count downstream of the filter to the particle count upstream.
Penetration is the ratio of the particle count downstream of the filter to the particle count upstream.
Efficiency of a HEPA filter:
Efficiency is the ratio of the number of particles retained by the filter to the number of the particles used to challenge the filter.
Efficiency is the ratio of the number of particles retained by the filter to the number of the particles used to challenge the filter.
Most Penetrating Particle Size (MPPS): It is a characteristic of a given filter at with this particle size filter has minimum efficacy.
Classification of HEPA filters EU norms EN 1822-1 by their retention at MPPS
H10 > 85 % (Total Retention)
H11 > 80%(Total Retention)
H12 > 99.5 % (Total Retention)
H13 >99.95 (Total Retention)
H14 >99.995% (Total Retention)
Classification of HEPA filters EU norms EN 1822-1 by their retention at MPPS
H10 > 85 % (Total Retention)
H11 > 80%(Total Retention)
H12 > 99.5 % (Total Retention)
H13 >99.95 (Total Retention)
H14 >99.995% (Total Retention)
HEPA filters standards.
ISO 14644 (1-9), introduced in 2000 most of European countries follow these standards
US FED STD-209E, 1992 USA.
JACA (Japan )
AS1386 (Australia)
ISO 14644 (1-9), introduced in 2000 most of European countries follow these standards
US FED STD-209E, 1992 USA.
JACA (Japan )
AS1386 (Australia)
How to clean a HEPA filter:
HEPA filters can be cleaned with a jet of air from down stream to the upstream direction so as to dislodge the accumulated particles, and then by a suction from reverse side if required (there should be a sanitisation procedure in place for suction clenear) followed by a jet of water gently, the quality of water is important in final rinsing. And the drying in a suitable place.
HEPA filters can be cleaned with a jet of air from down stream to the upstream direction so as to dislodge the accumulated particles, and then by a suction from reverse side if required (there should be a sanitisation procedure in place for suction clenear) followed by a jet of water gently, the quality of water is important in final rinsing. And the drying in a suitable place.
Decontamination of HEPA filters:
It is important to decontaminate HEPA filter when it is used in a clean room which handles vaccines , or a viruses ,bacteria.
HEPA must be decontaminated once it off line , it is decontaminated along with housing filters.
Decontamination procedure and chemicals are required to be chosen after consideration of microbial load and their resistance factors; validation of the entire process must be done. Integrity of a HEPA filter should be verified when it is being replaced again. Person carrying out the activity of decontamination of HEPA filter should be trained and provided a protective or a breathing apparatus so as to protect him from harmful affect of decontaminating chemicals like formaldehyde or H2O2 fumes. When HEPA filters are required to be disposed, they must be incinerated before disposal so as to avoid contamination of surrounding.
It is important to decontaminate HEPA filter when it is used in a clean room which handles vaccines , or a viruses ,bacteria.
HEPA must be decontaminated once it off line , it is decontaminated along with housing filters.
Decontamination procedure and chemicals are required to be chosen after consideration of microbial load and their resistance factors; validation of the entire process must be done. Integrity of a HEPA filter should be verified when it is being replaced again. Person carrying out the activity of decontamination of HEPA filter should be trained and provided a protective or a breathing apparatus so as to protect him from harmful affect of decontaminating chemicals like formaldehyde or H2O2 fumes. When HEPA filters are required to be disposed, they must be incinerated before disposal so as to avoid contamination of surrounding.
Related uscgmp link:
Related References:
1. Wikipedia, link use http://en.wikipedia.org/wiki/HEPA
4. American Society of Heating, Refrigerating and Air-Conditioning Engineers
5. Recommended Practice on HEPA and ULPA Filters
6. How HEPA filters work
5. Recommended Practice on HEPA and ULPA Filters
6. How HEPA filters work



Hello admin,
ReplyDeleteyou have given such a nice review here about the HEPA filters used in HVAC system. This type of review will give us a good information about it. It will be very helpful to all of us. Thank you very much for your post.
Final look is absolutely fantastic..I am also planning to do some renovation now that winter has passed. Gotta have a lot of small renovations to make which I know I can do myself. avoid air conditioner repair
ReplyDeleteThe HVAC experts the company owners are looking forward to should also provide installation of the new systems.home improvement contractors
ReplyDelete